Glucose and fructose are two of the most common types of sugar found in our diets. They are both monosaccharides, meaning they are simple sugars that cannot be broken down into smaller sugars. However, despite their similarities, the two have some key differences. This article explains the critical differences between glucose and fructose and how they impact the body’s properties and functions.
1. What is Glucose?
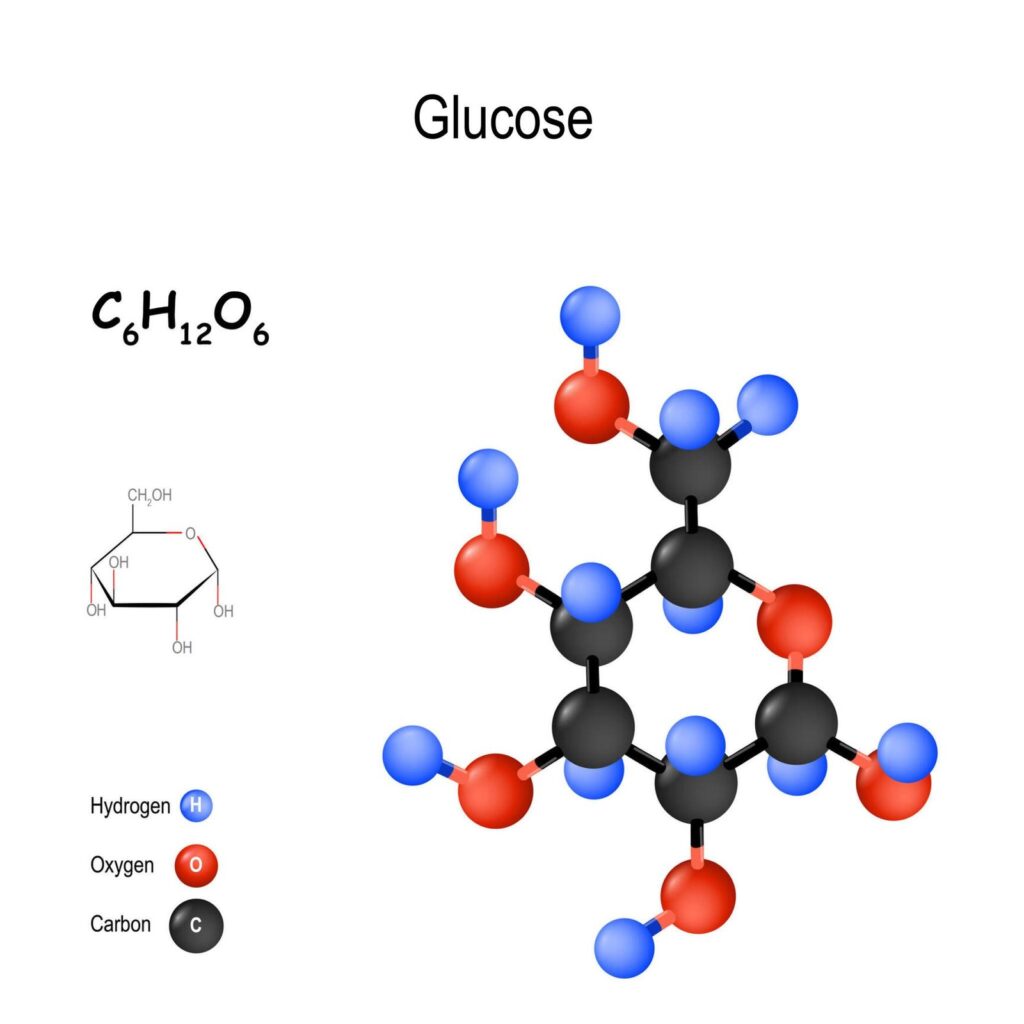
Glucose, or dextrose, is a simple sugar that is our bodies’ primary energy source. It is a monosaccharide, meaning it comprises a single sugar molecule. Glucose is found in many foods, including fruits, vegetables, and honey, and is also produced by our bodies through the breakdown of carbohydrates.
1.1 The Chemical Formula of Glucose
The chemical formula for glucose is C6H12O6, meaning it contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. This formula is the same for all monosaccharides, but the arrangement of these atoms differs, giving each sugar its unique properties.
1.2 The Structure of Glucose
The structure of glucose is a six-carbon ring with a hydroxyl group (-OH) attached to each carbon atom. This ring structure is known as a hexose, the most common form of glucose found in nature. The hydroxyl groups on the glucose molecule make it a polar molecule, meaning it can dissolve in water.
2. What is Fructose?
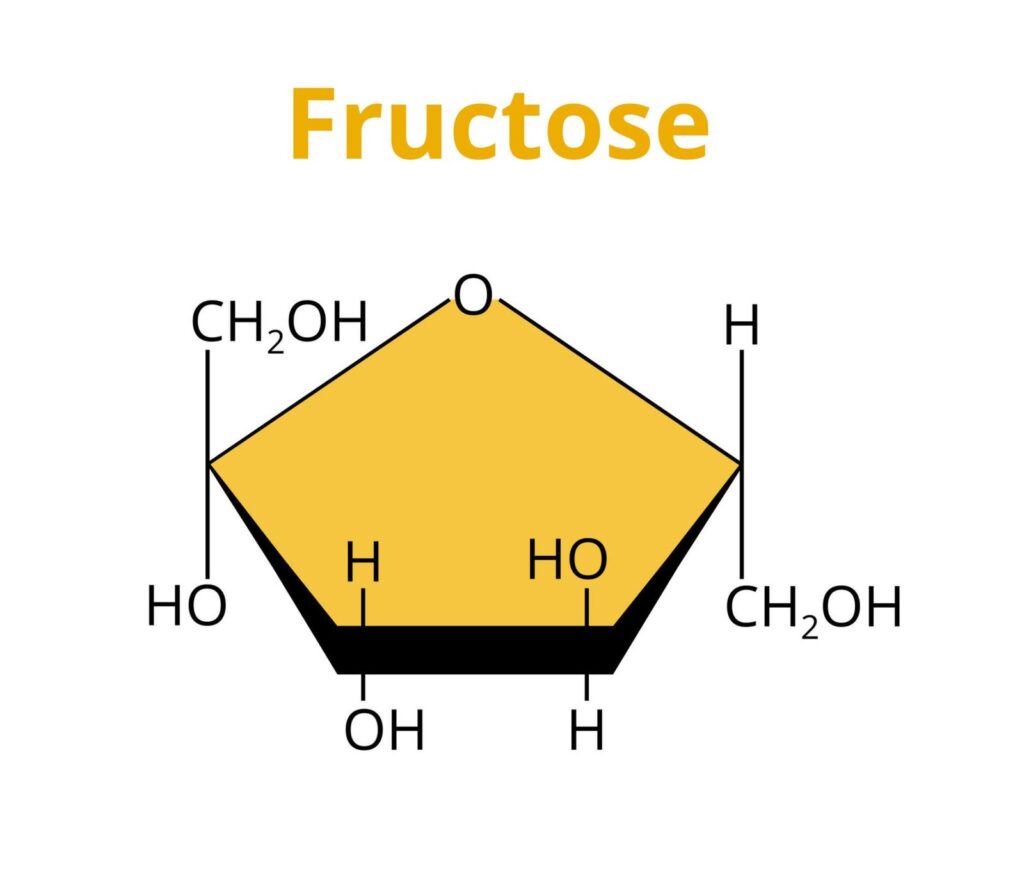
Fructose, or fruit sugar, is another simple sugar in many fruits, vegetables, and honey. It is also a monosaccharide with the same chemical formula as glucose (C6H12O6). However, the arrangement of atoms in fructose is different, giving it distinct properties from glucose.
2.1 The Structure of Fructose
The structure of fructose is a five-carbon ring with a ketone group (C=O) attached to one end and a hydroxyl group (-OH) attached to the other. This ring structure is known as a pentose, the most common form of fructose. The ketone group on the fructose molecule makes it a non-polar molecule, meaning it does not dissolve well in water.
3. The Structure of Glucose and Fructose
The primary distinction between glucose and fructose stems from their respective chemical compositions. Glucose and fructose have the same formula, but their arrangement of atoms makes them different.
3.1 Glucose Structure
Glucose is a hexose sugar possessing a ring structure comprising six carbon, twelve hydrogen, and six oxygen atoms. The carbon atoms are numbered from one to six, with the first carbon atom closest to the oxygen atom. The ring structure of glucose is known as a hexose, meaning it has six carbon atoms.
3.2 Fructose Structure
Fructose is also a six-carbon sugar with a ring structure. However, its atoms are arranged differently than glucose. Fructose and glucose have the same atoms, but they are arranged differently. The first carbon atom in fructose is closest to the oxygen atom. The other atoms are arranged differently, so fructose has a unique shape.
Byju’s is an Indian educational technology (edtech) company that offers online learning programs for students. It provides interactive video lessons, live classes, personalized learning paths, practice quizzes, and other educational resources across various subjects such as mathematics, science, and English.
Explore further to understand the structure of glucose and fructose by clicking here.
4. How the Structural Difference Affects Properties and Functions
The structural difference between glucose and fructose affects their properties and bodily functions in several ways.
4.1 Sweetness
One of the most noticeable differences between glucose and fructose is their sweetness. Fructose is much sweeter than glucose, with a sweetness level of 1.7 times that of glucose. The shape of fructose allows it to fit more easily into taste receptors on our tongues, making it taste sweeter.
4.2 Digestion and Absorption
The structural difference between glucose and fructose also affects how they are digested and absorbed. Glucose is easily absorbed into the bloodstream and used by our cells as energy. Fructose, on the other hand, is not used directly for energy. Instead, it is converted into glucose in the liver and then used as energy or stored as glycogen.
4.3 Metabolism

The structural difference between glucose and fructose also affects how they are metabolized. Glucose is metabolized quickly and provides a quick burst of energy. The body metabolizes fructose more slowly than glucose, so it does not offer the same rapid energy increase. Athletes often consume glucose during intense physical activity to replenish their energy levels quickly.
4.4 Effects on Blood Sugar Levels
Another significant difference between glucose and fructose is their effect on blood sugar levels. Glucose undergoes rapid absorption into the bloodstream, increasing blood sugar levels. This elevation prompts the secretion of insulin, a hormone pivotal in regulating blood sugar levels.
Fructose, on the other hand, is not absorbed as quickly, so it does not cause the same spike in blood sugar levels. Fructose is often recommended for people with diabetes or those trying to manage their blood sugar levels.
5. The Role of Glucose and Fructose in Our Diets
Both glucose and fructose play important roles in our diets, but it is essential to understand the differences between the two and consume them in moderation.
5.1 Glucose
Glucose serves as the primary energy source utilized by our bodies. It is quickly absorbed into our bloodstream and used by our cells for energy. Glucose is found in many foods, including fruits, vegetables, and grains, and is also produced by our bodies through the breakdown of carbohydrates.
5.2 Fructose
Fructose is also an energy source for our bodies, but the liver primarily metabolizes it. It is found in many fruits, vegetables, and honey and is used as a sweetener in many processed foods and beverages. While fructose is a natural sugar, consuming too much can negatively affect our health.
6. Glucose vs Fructose: Which is Better?
Both glucose and fructose have their unique properties and functions in the body. However, when it comes to which one is better, it ultimately depends on the individual’s needs and goals.
6.1 For Energy

If you need a quick burst of energy, glucose is the better option. It is quickly absorbed into the bloodstream and used by our cells as energy. However, fructose may be a better choice if you need sustained energy. It is metabolized more slowly and provides a longer-lasting source of energy.
6.2 For Blood Sugar Management

For those with diabetes or trying to manage their blood sugar levels, fructose may be a better option. It does not cause the same spike in blood sugar levels as glucose, making it a safer choice for those with diabetes.
Discover how to monitor blood sugar levels using CGM or flash technology. Click here to delve into this topic further.
6.3 For Weight Management
Regarding weight management, both glucose and fructose can play a role. Glucose is often recommended for athletes or those engaging in intense physical activity as it provides a quick burst of energy. Fructose is a better option for those trying to lose weight as it does not cause the same spike in blood sugar levels and may help regulate appetite.
7. Conclusion
In conclusion, the primary structural difference between glucose and fructose lies in their chemical structures. This difference affects their bodily properties and functions, including sweetness, digestion and absorption, metabolism, and effects on blood sugar levels. Both glucose and fructose have their unique roles in the body, and the best option ultimately depends on the individual’s needs and goals.
Explore further into food science-related topics by clicking here.

